News classification
News Center
Viwit Pharmaceuticals' Mirabegron API Receives Official Approval by China NMPA
- Categories:In 2024
- Author:
- Origin:
- Time of issue:2024-01-26 11:09
(Summary description)On January 23, 2024, Viwit Pharmaceuticals received the "Notice of Approval of Application for Listing of Chemical APIs" issued by China National Medical Products Administration (NMPA) for its own proprietary active pharmaceutical ingredient (API) Mirabegron, developed independently. The issued registration number is Y20220000096, and the corresponding notice number is 2024YS00039.
Viwit Pharmaceuticals' Mirabegron API Receives Official Approval by China NMPA
(Summary description)On January 23, 2024, Viwit Pharmaceuticals received the "Notice of Approval of Application for Listing of Chemical APIs" issued by China National Medical Products Administration (NMPA) for its own proprietary active pharmaceutical ingredient (API) Mirabegron, developed independently. The issued registration number is Y20220000096, and the corresponding notice number is 2024YS00039.
- Categories:In 2024
- Author:
- Origin:
- Time of issue:2024-01-26 11:09
- Views:
On January 23, 2024, Viwit Pharmaceuticals received the "Notice of Approval of Application for Listing of Chemical APIs" issued by China National Medical Products Administration (NMPA) for its own proprietary active pharmaceutical ingredient (API) Mirabegron, developed independently. The issued registration number is Y20220000096, and the corresponding notice number is 2024YS00039.
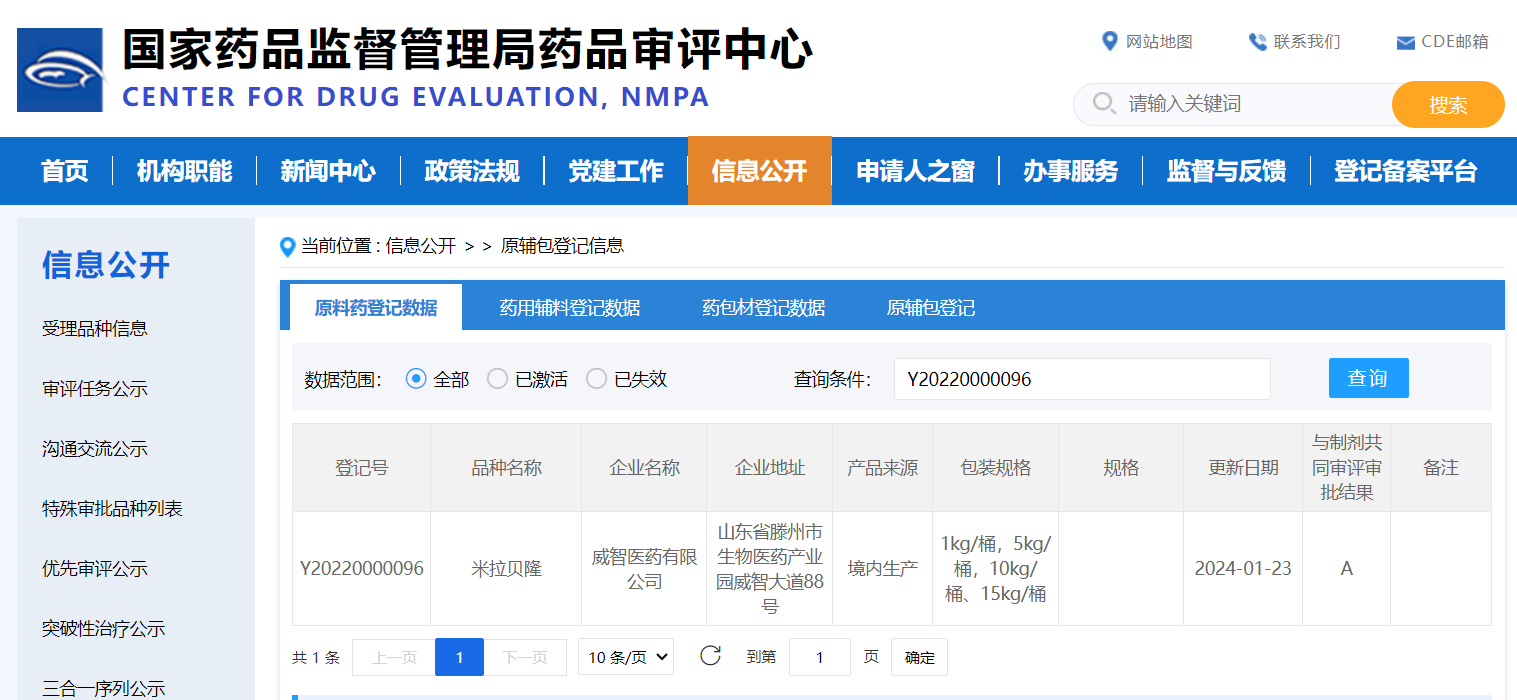
This notification affirms that Viwit's Mirabegron API aligns with the necessary criteria for domestic drug registration in China and has received approval for its utilization and commercialization in finalized dose formulations. Furthermore, the Mirabegron API facilitates the submission and registration processes for Mirabegron drug products in various countries. For example, in September 2022, the technical evaluation was completed and approved by the Egyptian Drug Authority. In January 2023, it received a registration certificate from South Korea's MFDS, followed by the successful completion of a remote GMP audit conducted by South Korea MFDS. In July 2023, the technical evaluation by the Taiwan Food and Drug Administration was completed, obtaining the Drug License from the Ministry of Health and Welfare in Taiwan, China.Viwit also submitted the US DMF and EU CEP Mirabegron API for approval.
Mirabegron dose formulations consist of extended-release tablets and granules. Functioning as a selective β3-adrenergic receptor agonist, it targets bladder tissues, leading to the relaxation of the smooth bladder muscle. The therapeutic benefits include addressing urgency, frequency, and/or urgency incontinence in adults diagnosed with Overactive Bladder.
The approval Mirabegron API represents a tangible outcome of Viwit Pharmaceuticals' integrated strategy for the development and manufacturing of drug substances as well as drug products. It is also a reflection of the company's comprehensive strength in API research and development capabilities, production, and quality management systems. In the future, the company will actively expand the sales of this API domestically and overseas to provide more choices for preparation customers.
Scan the QR code to read on your phone
VIWIT 沪ICP备12014533号-2 Terms of Use
