
News Center
Viwit Pharmaceuticals' Lidocaine Injection Receives Official Approval by China NMPA
Author:
Release Time:
2023-12-19
On December 18, 2023, Viwit Pharmaceuticals received the "Drug Supplementary Application Approval Notice" (Notice No: 2023B06224) issued by the China National Medical Products Administration for a proprietary finished dosage form, Lidocaine Hydrochloride for Injection (5ml: 0.1g). This regulatory endorsement attests to the product's alignment with the rigorous generic drug quality and efficacy consistency evaluation conducted by the NMPA. Viwit's commitment to research and development has met another milestone achievement, markedly reinforcing our company's competitive standing in the pharmaceutical landscape.
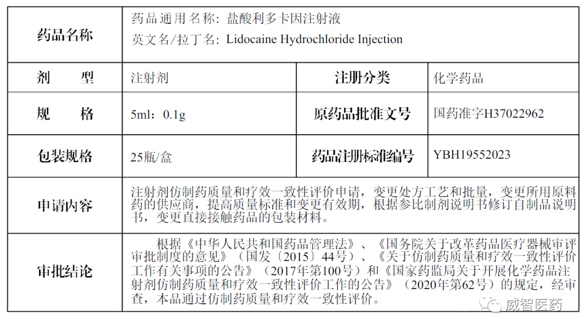
Lidocaine Hydrochloride Injection is indicated for several forms of local anesthesia and is widely recognized as a versatile anesthetic. Its primary applications include conduction anesthesia and epidural anesthesia. In 2022, the sales revenue of Lidocaine Hydrochloride Injection within the confines of Chinese public medical institutions surpassed $100 million, reflecting its significant market presence and utilization in clinical settings.

 中文
中文
 EN
EN