
News Center
Viwit Pharmaceuticals' Vildagliptin Tablets Receive Official Approval by China NMPA
Author:
Release Time:
2023-12-22
Viwit Pharmaceuticals is pleased to announce the acquisition of the Drug Registration Certificate (Certificate No: 2023S01974) from the National Medical Products Administration (NMPA) for a proprietary finished dosage form, Vildagliptin tablet. This signifies the official approval of this product in the Chinese market.
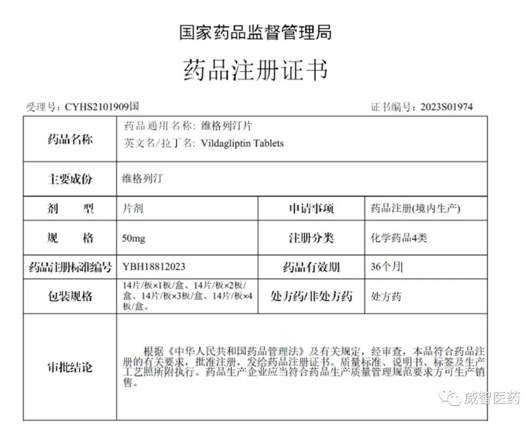
Vildagliptin Tablets are classified as Category 4 chemical drugs. Viwit Pharmaceuticals adheres to strict standards ensuring consistency with the original research in terms of quality and efficacy. In late 2022, the product successfully passed the on-site inspection for drug registration clinical trials conducted by the Center for Inspection of NMPA. In 2023, supplementary research was concluded, resulting in the timely acquisition of the Drug Registration Certificate. This achievement serves as comprehensive validation of the quality and efficacy of our company's product.
Vildagliptin, the second globally marked DPP-4 inhibitor, is utilized in reducing blood glucose concentrations among patients with Type II Diabetes, without inducing hypoglycemic events or influencing body weight. Vildagliptin was originally developed by Novartis and approved by the European Medicines Agency in 2007. Subsequently, it gained approval from NMPA to enter the domestic market in China in August 2011.
In 2017, Vildagliptin, along with four other gliptin class DPP-4 inhibitors, achieved inclusions in the China National Medical Insurance Catalog. Leveraging the distinctive advantages of DPP-4 inhibitors in the field of oral hypoglycemic pharmaceutical drugs, and coupled with the expiration of the original drug patent as well as the intense market competition, Vildagliptin tablets secured a position in the batch of China National Centralized Procurement Variety Catalog in 2020. This makes Vidagliptin the pioneering incorporation of a DPP-4 inhibitor in the centralized procurement initiative.
The successful market launch of Viwit Pharmaceuticals’ Vildagliptin Tablets provides another therapeutic option for patients within the target indications. The approval and subsequent market release of both Vildagliptin API and tablets represent a tangible outcome of Viwit Pharmaceuticals' integrated strategy for the development and manufacturing of drug substances as well as drug products. These consecutive approvals further fortify the company's commitment to the implementation of an integrated API-formulation strategy.
In the forthcoming period, Viwit Pharmaceuticals is committed to continuous technological innovation by augmenting investment, bolstering the cultivation of skilled technical talent, and advancing capabilities in the translation of research and development outcomes. Leveraging the industrial chain advantages of both APIs and drug products, Viwit Pharmaceuticals will actively compete in both domestic and international markets.

 中文
中文
 EN
EN