
News Center
News Center
Viwit Pharmaceutical's Phenylephrine Hydrochloride Injection Officially Receives Official Approval by China NMPA
Author:
VIWIT
Release Time:
2024-12-16
Recently, Viwit Pharmaceuticals received ‘Drug Registration Certificate’ issued by China National Medical Products Administration (NMPA) for independently developed Phenylephrine Hydrochloride Injection, the approval number is H20249727. This signifies that the product has received official approval for the Chinese market and been recognized as having passed the generic drug consistency evaluation.
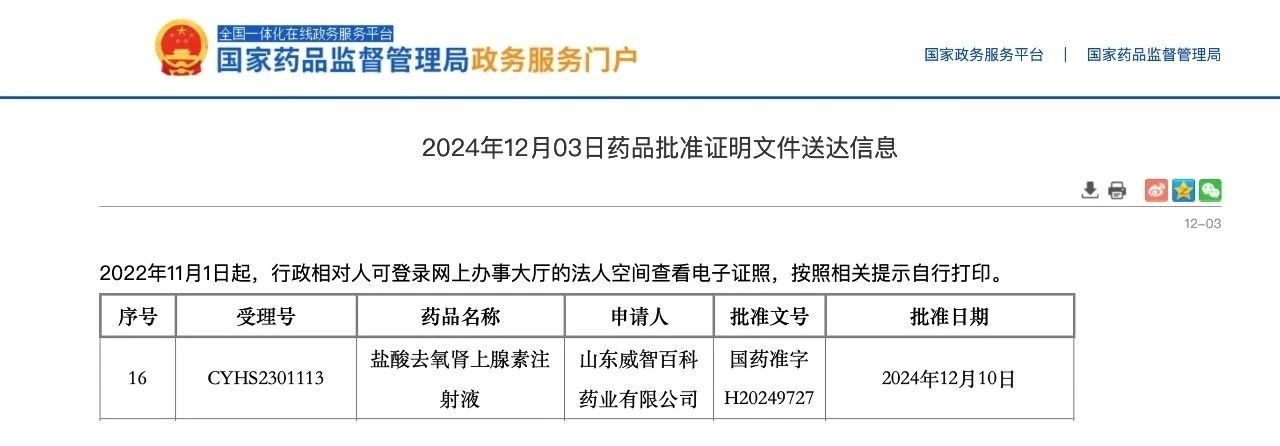
The approved strength for Phenylephrine Hydrochloride Injection is 5ml: 0.5mg, a ready-to-use formulation that does not require dilution before intravenous administration. It is indicated for the treatment of clinical hypotension caused by vasodilation during anesthesia. In addition to this product, Viwit Pharmaceutical has several cardiovascular products already available in the domestic market, including: Deslanoside Injection (Approval No. H61020256), Propyl gallate Injection (Approval Nos. H20050377, H20050378), and Nitroglycerin Sublingual Tablets which have also successfully launched in the U.S. market (ANDA 218583). Notably, the Nitroglycerin Sublingual Tablets are expected to gain formal domestic approval in Q1 2025, which is a significant step forward in Viwit’s cardiovascular drug R&D efforts.

Viwit Pharmaceutical will continue to uphold its corporate mission of “Innovation for a Better Life,” remaining committed to clinical needs and fostering innovation-driven research and development to provide patients with pharmaceutical products of greater clinical value.

 中文
中文
 EN
EN