
News Center
News Center
Viwit Pharmaceuticals’ Brimonidine tartrate and Timolol maleate eye drops receive Official Approval by China NMPA
Author:
VIWIT
Release Time:
2024-12-04
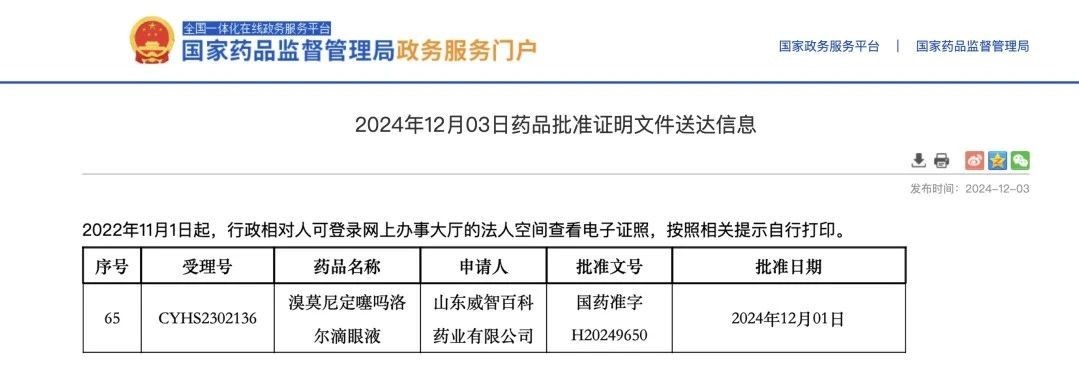
Recently, Viwit Pharmaceuticals received the "Drug registration certificate" issued by China National Medical Products Administration (NMPA) for Brimonidine Tartrate and Timolol Maleate Eye Drops. The approval number is H20249650. This signifies that the product has received official approval for the Chinese market and has successfully passed the generic drug consistency evaluation.
Brimonidine Tartrate and Timolol Maleate Eye Drops is a compound formulation composed of bromonidine tartrate and timolol maleate. The strength is 5 mL: 10.0 mg of bromonidine tartrate and 25.0 mg of timolol maleate (calculated as C₁₃H₂₄N₄O₃S), and it is used to lower intraocular pressure in adult patients with open-angle glaucoma or ocular hypertension. Glaucoma is an eye disease characterized by specific optic nerve damage and elevated intraocular pressure, often leading to blindness. Treatment methods include medication, laser therapy, and surgery. For open-angle glaucoma, drug therapy is the most fundamental approach, and combination therapies with drugs of different mechanisms are commonly used in clinical practice.
Bromonidine tartrate is an α2-adrenergic receptor agonist that reduces intraocular pressure by decreasing aqueous humor production and increasing outflow via the uveoscleral pathway. Timolol maleate is a non-selective β-adrenergic receptor antagonist with no significant endogenous sympathomimetic activity or local anesthetic effect, and no direct myocardial inhibitory effect. As a combined formulation, Brimonidine Tartrate and Timolol Maleate Eye Drops enhance intraocular pressure-lowering efficacy through complementary mechanisms, and compared to using the two components in separate eye drops, the combination reduces the frequency of administration and improves patient adherence.
Viwit Pharmaceuticals attaches great importance to drug research and development, with a focus on refractive errors, dry eye disease, anti-allergy, and glaucoma in the ophthalmic field, offering a wide range of products. In addition to the newly approved Brimonidine Tartrate and Timolol Maleate Eye Drops, other approved products include Olopatadine Hydrochloride Eye Drops (National Drug Approval No. H20233505) for allergic conjunctivitis, Bromonidine Tartrate Eye Drops (National Drug Approval No. H20243347) and Bimatoprost Eye Drops (National Drug Approval No. H20244103) for lowering intraocular pressure in open-angle glaucoma and ocular hypertension in different way.

Viwit Pharmaceuticals centers on customer needs and continuously enhances the innovation in the pharmaceutical and healthcare fields, striving to offer better health products and services. By pursuing the mission of “Innovation for a better life,” we aim to create more value for our customers, employees, shareholders, and society, contributing our intelligence and strength to a healthier and better life.

 中文
中文
 EN
EN