
News Center
News Center
Viwit Pharmaceuticals API Site Successfully Passed FDA cGMP On-site Audit with "No Action Indicated"
Author:
VIWIT
Release Time:
2024-09-23

From July 15 to 19, 2024, FDA inspectors conducted a five-day on-site cGMP (Current Good Manufacturing Practice) inspection of Viwit Pharmaceuticals Co., Ltd. (referred to as Viwit API manufacturing facility). Recently, the FDA's official website showed that Viwit's API manufacturing facility successfully passed the cGMP on-site audit with a perfect result of NAI, meaning "Zero 483". This is the second time Viwit's API manufacturing facility has successfully passed the U.S. FDA audit, further affirming Viwit’s robust and continuous production management and quality systems.
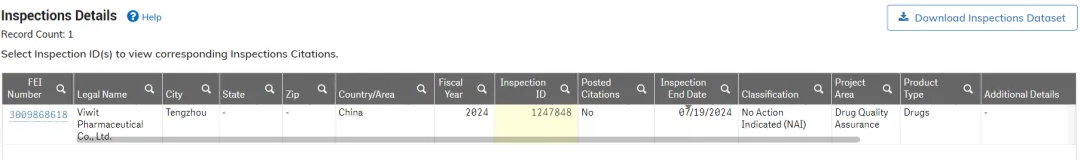
During the audit, FDA inspectors examined the company’s six major systems: quality management, production management, material management, facility management, laboratory control, and packaging and labeling systems. In the end, the audit concluded successfully with a perfect result of "Zero 483". This achievement is the result of the correct guidance and strong support from the corporate and the API division, the emphasis on quality management by the company's top management, and the unified efforts of all Viwit employees. It fully reflects Viwit’s dedication to quality. Whether it’s the production and quality teams working tirelessly on the front lines, or colleagues in Sales, Procurement, R&D, and various functional and support departments, everyone is embodying Viwit's core values of "customer-centricity and quality as life".

Successfully passing the FDA audit marks another milestone for Viwit's cGMP work and represents a new starting point for our cGMP efforts. Viwit will always maintain the pharmaceutical industry's commitment to quality, work diligently, thoroughly implement quality policies, and ensure quality work with the normalization of cGMP management. Meanwhile, Viwit will continue to build a high-quality, high-standard, and compliant API production base. Relying on continuous quality updates, technological innovation, and management improvement, Viwit aims to further enhance our international competitiveness in the API market, and support our products to expand globally.

 中文
中文
 EN
EN