
News Center
News Center
Viwit Pharmaceutical’s Lifitegrast API has completed registrations in both China and the US!
Author:
VIWIT
Release Time:
2024-08-14
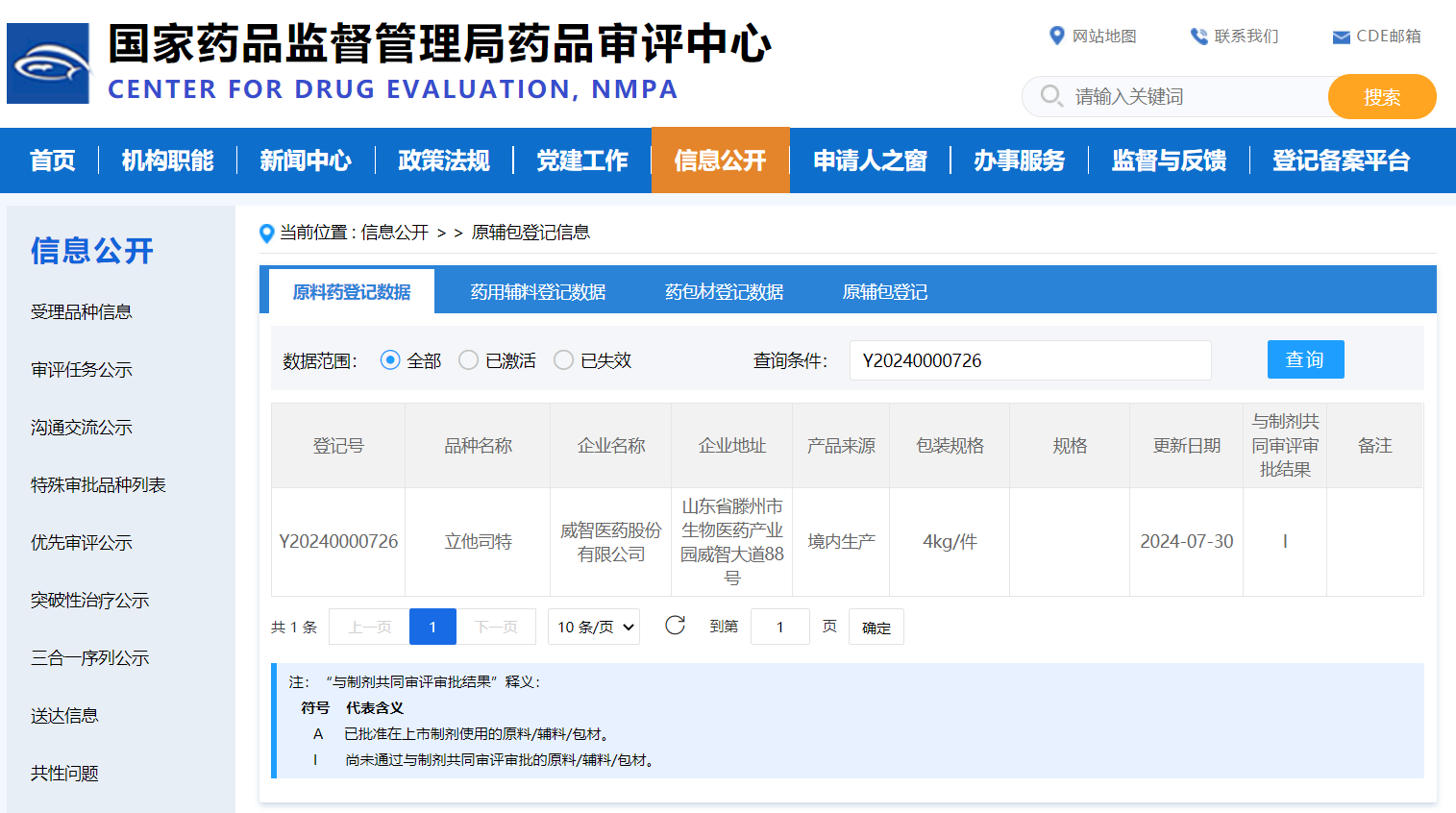
Recently, Viwit Pharmaceutical’s self-developed Lifitegrast API has completed the registrations in China and the United States. The API registration number is Y20240000726, and the US DMF number is 040172.
 |
|
The successful completion of these registrations in China and the United States signifies that Viwit pharmaceutical’s Lifitegrast API meets the research and regulatory requirements of clients in both markets. This achievement also ensures that the market demand for Lifitegrast eye drops is effectively addressed, thereby fully meeting the healthcare needs of patients.
Lifitegrast eye drops were initially developed by Shire Development LLC and marketed under the brand name Xiidra, with initial approval granted in the United States in July 2016. Since then, the product has been launched in Canada, Switzerland, South Korea, and several other countries. In China, however, Lifitegrast eye drops are still undergoing research and registration, with no products approved for market release.
Lifitegrast is a lymphocyte function-associated antigen-1 (LFA-1) antagonist used to treat symptoms of dry eye disease (DED). Lifitegrast binds to integrin LFA-1, a cell surface protein on leukocytes, blocking the interaction between LFA-1 and its cognate ligand, intercellular adhesion molecule 1 (ICAM-1). This action inhibits T-cell-mediated cytokine secretion, thereby exerting an anti-inflammatory effect.
Dry eye disease (DED), also known as keratoconjunctivitis sicca (KCS), manifests with symptoms such as dry eyes, foreign body sensation, itching, photophobia, redness, blurred vision, and eye fatigue. DED can lead to keratitis and decreased vision, and severe cases may result in corneal ulcers or even blindness. It has become a significant public health issue.
Looking ahead, Viwit Pharmaceutical will maintain its commitment to being "customer-centered and quality-focused". We will continue to advance our innovation through research and development in the pharmaceutical and healthcare sectors. We strive to provide high-quality health products and services, actively fulfilling our corporate mission of "innovating for a better life", and continuously creating more value for our customers, employees, shareholders, and society, contributing our wisdom and strength to a healthier and better life!

 中文
中文
 EN
EN
