
News Center
News Center
Viwit Pharmaceutical's Fosaprepitant Dimeglumine API Receives Official Approval from NMPA
Author:
VIWIT
Release Time:
2024-07-12
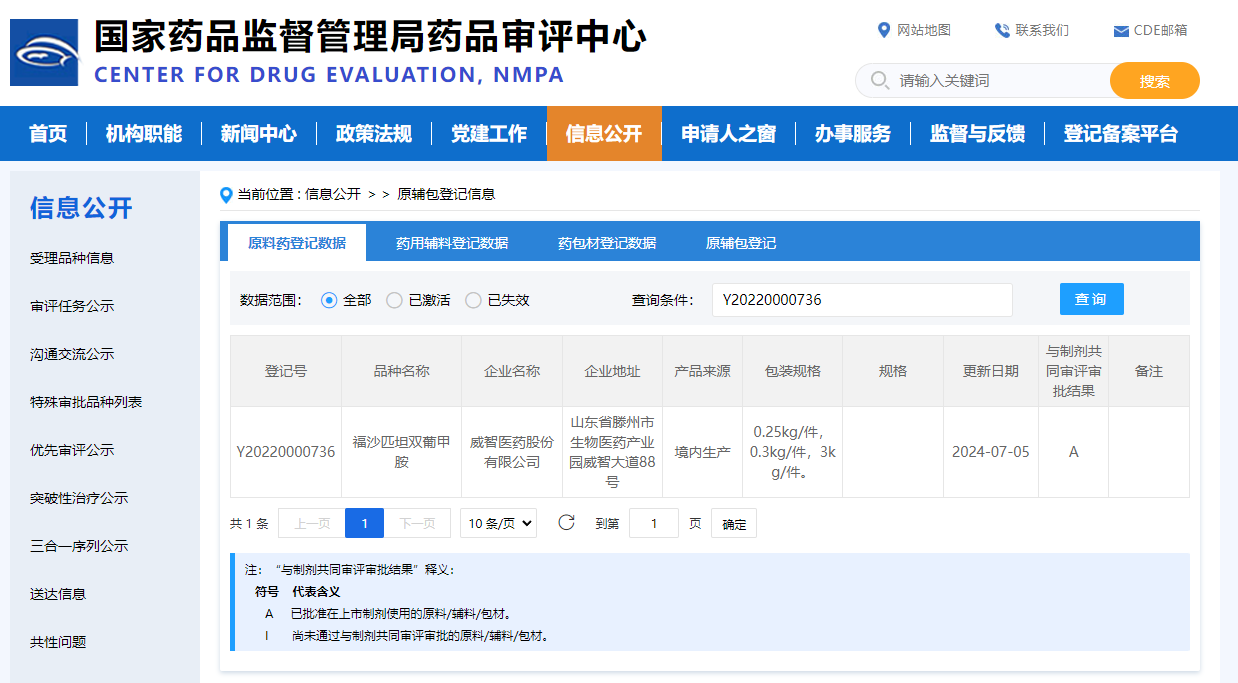
On July 5, 2024, Viwit Pharmaceitical’s independently developed Fosaprepitant Dimeglumine API received the "Notice of Approval of Application for Listing of Chemical APIs" issued by National Medical Products Administration (NMPA). The registration number is Y20220000736, and the notice number is 2024YS00664.
Fosaprepitant Dimeglumine is a water-soluble phosphorylated prodrug of Aprepitant. After intravenous injection, it rapidly converts to Aprepitant in the body through a phosphorylation pathway. Aprepitant selectively blocks the neurokinin-1 (NK-1) receptor, thereby inhibiting chemotherapy-induced vomiting. It is mainly used to prevent acute and delayed nausea and vomiting caused by initial and repeat use of moderately to highly emetogenic chemotherapy drugs. In October 2023, Aprepitant API, also independently developed by Viwit Pharmaeutical, was approved for marketing in China. The successive approvals of these products consistently demonstrate the company's comprehensive R&D capabilities.
Viwit Pharmaceutical will continue to adhere to a customer-centric approach, enhancing the level, depth, and breadth of innovation in the pharmaceutical health field through R&D and innovation. We strive to provide higher quality health products and services, fulfilling the mission of "innovation for a better life," and continually creating more value for customers, employees, shareholders, and society. We will contribute our intelligence and strength to a healthier, better life!

 中文
中文
 EN
EN